Abstract
Background:
Current guidelines recommend low molecular weight heparin (LMWH) over vitamin K antagonists for the treatment of cancer associated thrombosis (CAT). In recent years, several direct oral anticoagulants (DOACs) have been approved for the treatment of venous thromboembolism (VTE). However, these drugs have not been prospectively studied in cancer patients, a population with a high risk of recurrent thrombosis. It is known that certain types of cancer are more thromobogenic than others, further complicating the issue. Despite the lack of data, many providers are using DOACs to treat CAT due to the ease of administration and lower cost compared to LMWH. We sought to analyze prescription patterns of anticoagulants with particular attention to the choice of anticoagulant in relation to cancer type.
Methods:
Data were obtained from the Explorys (IBM Watson, Inc.) platform, an externally validated, de-identified dataset which pools data from multiple US healthcare organizations. To conserve de-identification, all results are rounded to nearest 10. We created cohorts and analyzed the temporal relationship of anticoagulants prescribed after a diagnosis of cancer. Data available for each cohort included the number of patients treated with an anticoagulant(s) over the past 1 year, 3 years, or "ever" treated. Increased use of an anticoagulant was arbitrarily defined as ≥70% of patients being treated within the past 3 years vs ever treated; decreased use was arbitrarily defined as ≤30%. We included all patients age ≥ 18 with history of malignant neoplastic disease. We defined cancer risk based on the Khorana VTE risk model.
Results:
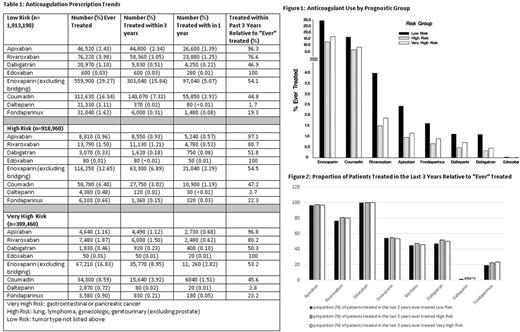
The study population comprised 3,231,610 cancer patients. Of these, 59% (n=1,913,190) were classified as having low risk cancers. High risk and very high risk comprised 28% (n=918,960) and 12% (n=399,460), respectively (Table 1). We found 526,290 (16.3%) patients received at least one anticoagulant during the study period. Enoxaparin was the most frequently prescribed anticoagulant regardless of risk category but there was a trend for increased use of DOACs in the low risk cancer types vs high and very high risk cancer types (7.5% vs 2.8% vs 3.5%, respectively p<0.001). Figure 1 shows anticoagulation prescription pattern by prognostic group. Over the past 3 years, there has been an increased use of apixaban, rivaroxaban and edoxaban with decreased use of dalteparin and fondaparinux (Figure 2).
Conclusions:
The standard treatment recommendation for cancer associated thrombosis remains treatment with LMWH, however, many providers opt for the new oral agents. Despite our data's limitations of anticoagulation crossover and the approximation with regard to time trends, we see a pattern of increased use of DOACs in the cancer population. We note the interesting finding that DOAC use is less common in patients with high or very high risk tumor types which suggests providers are wary of deviating from the standard of care in such patients.
Khorana: Janssen Scientific Affairs, LLC: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Halozyme: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Amgen: Consultancy, Research Funding; Leo: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal